DELTA2 guidance on choosing the target difference and undertaking and reporting the sample size calculation for a RCT_Review
DELTA2 guidance on choosing the target difference and undertaking and reporting the sample size calculation for a RCT_Review
Information
link: https://www.bmj.com/content/363/bmj.k3750
Introduction
- RCT(randomised controlled trials)
- Properly conducted, randomised controlled trials are considered to be the best method for
- assessing the comparative clinical efficacy and
- effectiveness of healthcare interventions,
- providing a key source of data for estimating cost effectiveness
- Properly conducted, randomised controlled trials are considered to be the best method for
- priori sample size calculation
- Central to the design of a randomised controlled trial
- ensures that the study has a high probability of achieving its prespecified objective
- The difference between groups
- used to calculate a sample size for the trial (known as the target difference) is
- the magnitude of difference in the outcome of interest that
- the randomised controlled trial is designed to reliably detect
- The DELTA2 project,
- commissioned by the United Kingdom’s Medical Research Council/National Institute for Health Research Methodology Research Programme and
- aimed to produce updated guidance
- for researchers and funders
- on specifying and reporting the target difference (the effect size)
- in the sample size calculation of a randomised controlled trial.
- we summarise
- the process of developing the new guidance, as well as
- the relevant considerations, key messages, and
- recommendations for researchers determining and reporting
- sample size calculations for randomised controlled trials
Box 1: DELTA2 recommendations for researchers undertaking a sample size calculation and choosing the target difference
- Begin by searching for relevant literature to inform the specification of the target difference.
- Relevant literature can:
- relate to a candidate primary outcome or the comparison of interest, and;
- inform what is an important or realistic difference
- for that outcome, comparison, and population.
- Relevant literature can:
- Candidate primary outcomes should be considered in turn, and
- the corresponding sample size explored.
- Where multiple candidate outcomes are considered,
- the choice of the primary outcome and target difference should be based on
- consideration of the views of relevant stakeholder groups (eg, patients), as well as
- the practicality of undertaking such a study with the required sample size.
- The choice should not be based solely on
- which outcome yields the minimum sample size.
- Ideally, the final sample size will be sufficient for all key outcomes,
- although this is not always practical.
- the choice of the primary outcome and target difference should be based on
- The importance of observing a particular magnitude of a difference in an outcome,
- with the exception of mortality and other serious adverse events,
- cannot be presumed to be self evident.
- Therefore, the target difference for all other outcomes needs
- additional justification to infer importance to a stakeholder group.
- The target difference for a definitive trial (eg, phase III) should be one
- considered to be important to at least one key stakeholder group.
- The target difference does not necessarily have to be the minimum value
- that would be considered important
- if a larger difference is considered a realistic possibility or would be necessary to alter practice.
- Where additional research is needed to inform what would be an important difference,
- the anchor and opinion seeking methods are to be favoured.
- The distribution method should not be used.
- Reference for the anchor and the distribution method
- Specifying the target difference based solely on a
- standardised effect size approach should be considered a last resort,
- Where additional research is needed to inform what would be a realistic difference,
- the opinion seeking and the review of the evidence base methods are recommended.
- Pilot trials are typically too small to inform what would be a realistic difference and
- primarily address other aspects of trial design and conduct.
- Use existing studies to inform the value of key nuisance parameters
- that are part of the sample size calculation.
- For example, a pilot trial can be used
- to inform the choice of the standard deviation value for a continuous outcome and
- the control group proportion for a binary outcome,
- along with other relevant inputs such as the amount of missing outcome data.
- Sensitivity analyses, used in the sample size calculation, should be carried out.
- which consider the effect of uncertainty around key inputs
- (eg, the target difference and the control group proportion for a binary outcome)
- which consider the effect of uncertainty around key inputs
- Specification of the sample size calculation, including the target difference,
- should be reported according to the guidance for reporting items (see table 1)
- when preparing key trial documents (grant applications, protocols, and result manuscripts).
- should be reported according to the guidance for reporting items (see table 1)
Development of the DELTA 2 guidance
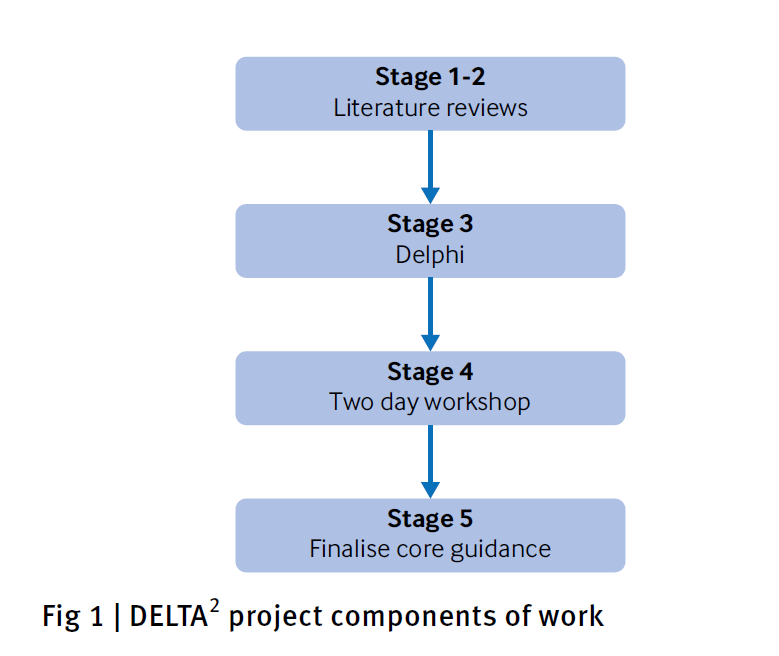
- The DELTA2 guidance is the culmination of a five stage process
- The core guidance was provisionally
- finalised in October 2017 and
- reviewed by the funders’ representatives for comment
- (Methodology Research Programme advisory group).
- The guidance was further revised and finalised in February 2018.
- The full guidance document incorporating case studies and relevant appendices is available here.
- Further details on the findings of the Delphi study and the wider engagement with stakeholders are reported elsewhere.
- The guidance and key messages are summarised in the remainder of this paper.
The target difference and sample size calculations in randomised controlled trials
- The role of the sample size calculation is
- to determine how many patients are required
- for the planned analysis of the primary outcome to be informative
- It is typically achieved by
- specifying a target difference for the key (primary) outcome
- that can be reliably detected and the required sample size calculated
- The precise research question that the trial is primarily set up to answer
- will determine what needs to be estimated in the planned primary analysis,
- which is known formally as the “estimand”
- The target difference should be a difference that is appropriate for that estimand.
- The target difference should be viewed as important by
- at least one (and preferably more) key stakeholder groups—
- that is, patients, health professionals, regulatory agencies, and healthcare funders.
- In practice, the target difference is not always formally considered and
- in many cases appears, at least from trial reports, to be determined on convenience, the research budget, or some other informal basis.
- at least one (and preferably more) key stakeholder groups—
- The target difference can be expressed as an
- absolute difference
- (eg, mean difference or difference in proportions) or
- relative difference
- (eg, hazard or risk ratio)
- is also often referred to, rather imprecisely, as the trial “effect size
- absolute difference
- Statistical calculation of the sample size is far from an exact science
- Firstly, investigators typically make assumptions
- that are a simplification of the anticipated analysis.
- For example, the impact of adjusting for baseline factors is difficult to quantify upfront,
- and even though the analysis is intended to be an adjusted one
- (such as when randomisation has been stratified or minimised),
- the sample size calculation is often conducted on the basis of an unadjusted analysis.
- and even though the analysis is intended to be an adjusted one
- Secondly, the calculated sample size can be sensitive to the assumptions made in the calculations
- a small change in one of the assumptions can lead
- to substantial change in the calculated sample size.
- Often a simple formula can be used to calculate the required sample size.
- a small change in one of the assumptions can lead
- Firstly, investigators typically make assumptions
- it is necessary for researchers to balance
- the risk of incorrectly concluding that there is a difference (Type I error)
- when no actual difference between the treatments exists,
- with the risk of failing to identify a meaningful treatment difference when the treatments do differ(Type II error)
- Under the conventional approach, referred to as the statistical hypothesis testing framework
- the probabilities of these two errors are controlled by setting
- the significance level (type I error) and
- statistical power (1 minus type II error) at appropriate levels
- (typical values are two sided 5% significance and 80% or 90% power, respectively).
- Once these two inputs have been set, the sample size can be determined given
- the magnitude of the between group difference in the outcome it is desired to detect
- (the target difference).
- the magnitude of the between group difference in the outcome it is desired to detect
- The calculation (reflecting the intended analysis) is conventionally done
- on the basis of testing for a difference of any magnitude
- the probabilities of these two errors are controlled by setting
- the risk of incorrectly concluding that there is a difference (Type I error)
- A key question of interest is what magnitude of difference can be ruled out.
- The expected (predicted) width of the confidence interval can be determined
- for a given target difference and sample size calculation,
- The required sample size is very sensitive to the target difference.
- The expected (predicted) width of the confidence interval can be determined
- In more complex scenarios, simulations can be used
- It is prudent to undertake sensitivity calculations to assess
- the potential effect of misspecification of key assumptions such as
- the control response rate for a binary outcome or
- the anticipated variance of a continuous outcome
- the potential effect of misspecification of key assumptions such as
- It is prudent to undertake sensitivity calculations to assess
Specifying the target difference for a randomised controlled trial
- the specification of the target difference for a randomised controlled trial,
- a series of recommendations is provided in box 1 and table 1.
- Seven broad types of methods can be used
- to justify the choice of a particular value as the target difference, which are summarised in box 2
- Box 2: Methods that can help inform the choice of the target difference
- Methods that inform what is an important difference
- Anchor
- using either a patients’ or health professional’s judgment to define what an important difference is
- by comparing a patients’ health before and after treatment and then
- linking this change to participants who showed improvement or deterioration using a more familiar outcome
- Distribution
- determine a value based on distributional variation
- use a value that is larger than the inherent imprecision in the measurement and therefore
- likely to represent a minimal level needed for a noticeable difference
- Health economic
- use the principles of economic evaluation
- compare cost with
- health outcomes and
- define a threshold value for the cost of a unit of healt effect that a decision maker is willing to pay
- compare cost with
- to estimate the overall incremental net benefit of one treatment versus the comparator
- use the principles of economic evaluation
- Standardised effect size
- the magnitude of the effect on a standardised scale defines the value of the difference
- For continuous outcome, the standardised difference can be used
- e.g., Cohen’s d effect size
- the mean difference/the S.D
- e.g., Cohen’s d effect size
- For binary or survival(time-to-event) outcome, odds, risk or hazard ratio can be used
- no widely recognised cutoff points exist
- Anchor
- Methods that inform what is a realistic difference
- Pilot Study
- to guide expectations and determine an appropriate target difference for the trial
- Phase 2 study could be used to inform Phase 3 study
- Pilot Study
- Methods that inform what is an important or a realistic difference
- Opinion seeking
- the target difference can be based on opinions elicited from health professionals, patients, or others
- Possible approaches
- forming a panel of experts
- surveying the membership of a professional or patient body
- intervewing individuals
- Review of evidence base
- the target difference can be derived from current evidence on the research question
- Ideally, from a systematic review or meta-analysis of randomised controlled trials
- In the absence of randomised evidence, evidence from observational studies could be used in a similar manner
- Opinion seeking
- Methods that inform what is an important difference
- Target difference should always be both important and realistic,
- which would seem particularly apt
- when designing a definitive (phase 3) superiority randomised controlled trial.
- In a sample size calculation for a randomised controlled trial,
- the target difference between the treatment groups strictly relates to
- a group level difference for the anticipated study population.
- the target difference between the treatment groups strictly relates to
- which would seem particularly apt
Reporting the sample size calculation
- The approach taken to determine the sample size and the assumptions made should be clearly specified.
- all the inputs and formula or simulation results,
- so that it is clear what the sample size was based on.
- critical for reporting transparency,
- allows the sample size calculation to be replicated, and
- clarifies the primary (statistical) aim of the study.
- all the inputs and formula or simulation results,
- approach with a standard trial design (1:1 allocation, two arm, parallel group, superiority design) and unadjusted statistical analysis,
- the core items are
- the primary outcome, the target difference appropriately specified according to
- the outcome type,
- the associated nuisance parameter
- (that is, a parameter that, together with the target difference, uniquely specifies the difference on the original outcome scale
- eg, the event rate in the control group for a binary primary outcome), and
- the statistical significance and power
- the primary outcome, the target difference appropriately specified according to
- the core items are
- More complicated designs can have additional inputs
- such as the intracluster correlation for a cluster randomised design
- When the sample size calculation deviates from the conventional approach,
- whether by research question or statistical framework,
- the core reporting set can be modified to provide
- sufficient detail to ensure that the sample size calculation is reproducible and
- the rationale for choosing the target difference is transparent.
- If the sample size is determined on the basis of a series of simulations,
- this method should be described in sufficient detail
- to provide an equivalent level of transparency and assessment
- this method should be described in sufficient detail
Discussion
- Researchers are faced with a number of difficult decisions when designing a randomised controlled trial, the most important decisions are
- The choice of trial design,
- primary outcome, and
- sample size
- The sample size is largely driven by
- the choice of the target difference
- The DELTA2 guidance provides help on
- specifying a target difference and
- undertaking and reporting the sample size calculation for a randomised controlled trial.
- The guidance was developed in response to a growing recognition from funders, researchers, and other key stakeholders (such as patients and the respective clinical communities) of a
- real need for practical and accessible advice to inform a difficult decision.
- The key message for researchers is the need
- to be more explicit about the rationale and
- justification of the target difference
- when undertaking and reporting a sample size calculation.
- Increasing focus is being placed on the target difference
- in the clinical interpretation of the trial result,
- whether statistically significant or not.
This post is licensed under CC BY 4.0 by the author.